The field of nuclear medicine is the application radionuclides in the diagnostic and treatment of diseases. The background for this is nuclear physics and radioactivity.
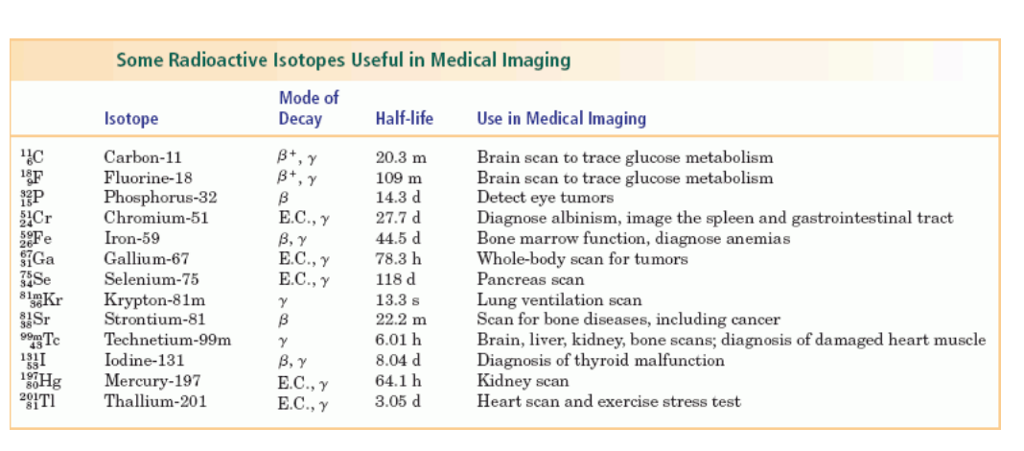
Atomic nucleus is made up of protons and neutrons. The number of protons usually depicts the atomic number(Z), but the sum of the numbers of protons and neutrons is the mass number(A). The stomic and mass numbers are useful parameters as we use tables with data on the atoms of different elements. In some situations, nuclei do have the same atomic number but different mass number, making them isotopes. Radioisotopes like technetium-99, iodine-131, rubidium-82 and thallium-201 and some others are used a lot in nuclear medicine.
A typical nucleus has radius, r = (1.2×10^-15)(A^0.333) m, dependent on its mass number. The strong nuclear force overshadows the repulsive electrostatic force, and hence energy equal to its binding energy is needed to separate it into its constituents. The binding energy obtained from Einsteins mass-energy relationship is the product of the nucleus mass defect and the square of the speed of light in the vacuum. Generally, the sum of the masses of a nucleus parts exceeds that of the parent., and the difference is the mass defect. The masses are measured in atomic mass units with equivalents in kilogram and MeV (mega-electron-volt).
For nucleus with Z greater than 82, unstable lead them to undergo radioactive decay by either alpha decay, beta decay or gamma decay. Alpha decay by nucleus rich in protons reduces A by 4 and Z by 2. For those with a lot of neutrons, beta decay occurs with the emission of an electron or its antiparticle, positron. When an electron is emitted A remains the same but Z increases by 1. If a positron is emitted, A remains the same bu Z decreases by 1. Gamma decay is accompanied by the emission of energetic photons with no change to either A and Z of the nucleus. In some of these decay processes, new elements are formed, and if they are unstable, the chain of event continues until stability is achieved. The time needed to decay to half its original size or number is known as the half-life (T-0.5) The product of the half life and the decay constant is 0.693. The activity (measured in becquerel, Bq) of the nucleus is the number of radioisotope nuclei times its decay constant. The number of remaining isotopes or activity after a period of time are modeled using exponential decay function as N(t) = N’e^(-decay constant x time) or A(t) = A’e^(-decay constant x time).
A popular unit for activity is curie (Ci), and 1 Ci = 3.7 x 10^10 Bq.
To put things in perspective, it is important to note that the natural radioactivity in us is about 10 nCi, scanning techniques is between 10 microCi to 10 mCi, and those in the gamma ray soruces in radiotherapy is about 1000 Ci. The table below gives the decay mode, half life and applications of some radioisotopes in medicine.